biobank
Première banque française d’allogreffes osseuses, BIObank est une entreprise indépendante qui bénéficie de la certification ISO 13485 pour des allogreffes osseuses et met son expertise au service de la santé depuis plus de 20 ans.
Agréée par l’Agence nationale de sécurité du médicament (ANSM), l’entreprise assure l’ensemble des activités d’une banque de tissus, du recueil des têtes fémorales jusqu’à la transformation des greffons osseux.


principe et avantages du procédé supercrit®
Le procédé Supercrit® est une méthode basée sur la délipidation du tissu osseux par le CO2 à l'état supercritique, associée à une oxydation chimique des protéines résiduelles dans les pores du tissu spongieux.
Par sa très faible viscosité et son puissant pouvoir solvant, le CO2 supercritique présente une efficacité délipidante incomparable et sans agression sur la matrice osseuse.
Découvrir Supercrit®l'adn biobank
Qualité
BIOBank s'attache à offrir des produits et des services d'une qualité irréprochable.
Rigueur
En appliquant les normes et les règlements les plus stricts, BIOBank garantit la sécurité de ses produits.
Innovation
Pour répondre aux besoins actuels et futurs des chirurgiens et des patients, BIOBank investit toujours plus dans la recherche et le développement.
Certifications
BIOBank est autorisée par l'ANSM comme établissement de tissus conformément aux dispositions de la directive européenne 2004/23/CE.
BIOBank est certifiée ISO 13485 et enregistrée par la FDA.
la sécurité au centre de notre processus
Pour assurer la sécurité des receveurs, nous contrôlons toute la chaîne d'implantation des greffons. Notre démarche transparente assure une qualité constante des tissus et des produits transformés.
Les activités de BIOBank font l'objet d'une autorisation délivrée par l'ANSM. De même, les greffons osseux bénéficient d'une autorisation unique de mise sur le marche délivrée par l'ANSM. BIOBank dispose également d'une autorisation d'exportation de ses greffons.
01
Recueil et sélection
02
Transport
03
Transformation et conditionnement
04
Distribution et conservation
06
Traçabilité
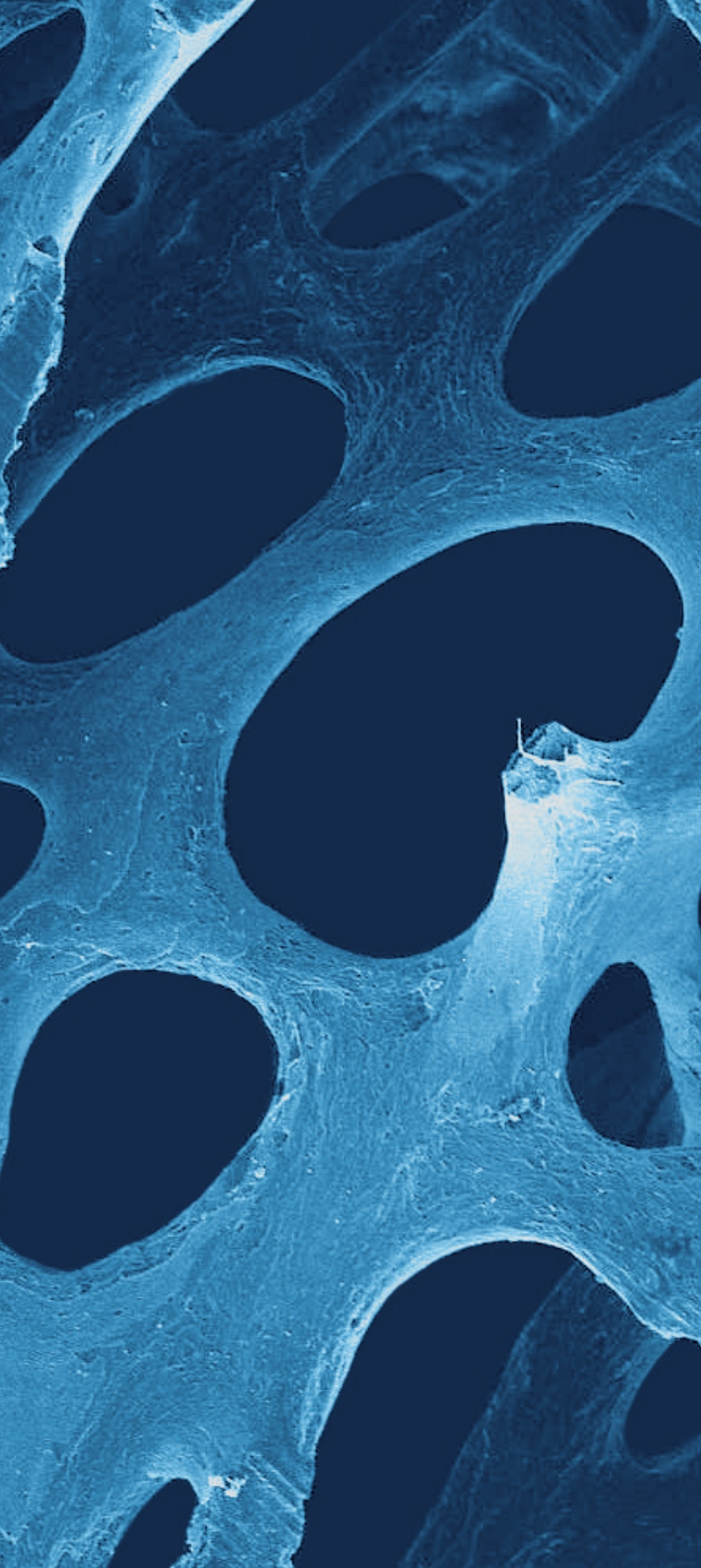
l'innovation
au cœur de biobank
Technologie Supercrit®
L'innovation est au cœur du projet d'entreprise de BIOBank. En étant la première banque de tissus au monde à utiliser la technologie brevetée des fluides supercritiques pour assurer le dégraissage et la viro-inactivation d'allogreffes osseuses, BIOBank s'est placée dès son lancement dans une démarche innovante.
Conditionnement innovant
La poudre d’os est proposée dans une seringue au design spécifiquement développé. Cette innovation permet au chirurgien de réaliser la réhydratation du tissu osseux dans un système semi-clos, gage d’une asepsie optimale, et de délivrer la poudre réhydratée directement dans le site de greffe. Cette innovation trouve des applications performantes pour les petits comblements en chirurgie orale comme pour les arthrodèses en chirurgie du rachis.
Greffons sur mesure
Par la mise en œuvre de technologies sophistiquées, autour de l’analyse d’images scanner, la modélisation et l’impression 3D et de l’usinage sur centre numérique, BIOBank est la seule banque de tissus à offrir des greffons osseux pré-usinés, parfaitement adaptés à l’anatomie osseuse du patient. Cette approche constitue une véritable révolution pour la qualité de la reconstruction osseuse et le confort des patients par la minimisation du temps chirurgical et des suites opératoires.
Futurs développements
En partenariat scientifique avec plusieurs laboratoires académiques, BIOBank investit dans de nouvelles formes et formulations à la bioactivité augmentée. BIOBank s’est donné comme mission de toujours rester à la pointe de l’innovation pour mettre à la disposition des chirurgiens des produits à forte valeur ajoutée scientifique, au bénéfice de la performance et de l’efficacité clinique.




