Des performances inégalées en termes de sécurité et de respect de la matrice osseuse.
Supercrit® : La combinaison optimale du dégraissage par le CO2 supercritique et d'une oxydation chimique douce des protéines résiduelles.
sUPERCRIT®, LE TRAITEMENT DU TISSU OSSEUX
Diverses méthodes de traitement ont été développées dans le but de dévitaliser le tissu osseux et le rendre propre aux trois propriétés essentielles attendues : sécurisation virale active validée (et non plus par la seule sélection des donneurs), conservation à température ambiante dans un état déshydraté et ostéoconduction améliorée par le nettoyage des travées osseuses.
L’enjeu principal est de dégraisser le tissu osseux spongieux qui contient de nombreux adipocytes dans ses cavités médullaires et de potentiels agents pathogènes. Cette fraction lipidique représente en effet près de 60 % en masse du tissu osseux spongieux frais. Son élimination est rendue d’autant plus difficile que le réseau trabéculaire est dense et épais (Figure 1).
BIOBank est la première banque de tissus à avoir développé et utilisé un fluide performant et non toxique, le CO2 à l’état supercritique. Le savoir-faire acquis et développé par BIOBank lui permet de maîtriser cette technologie qui présente des performances inégalées en termes de sécurité et de respect de la matrice osseuse.


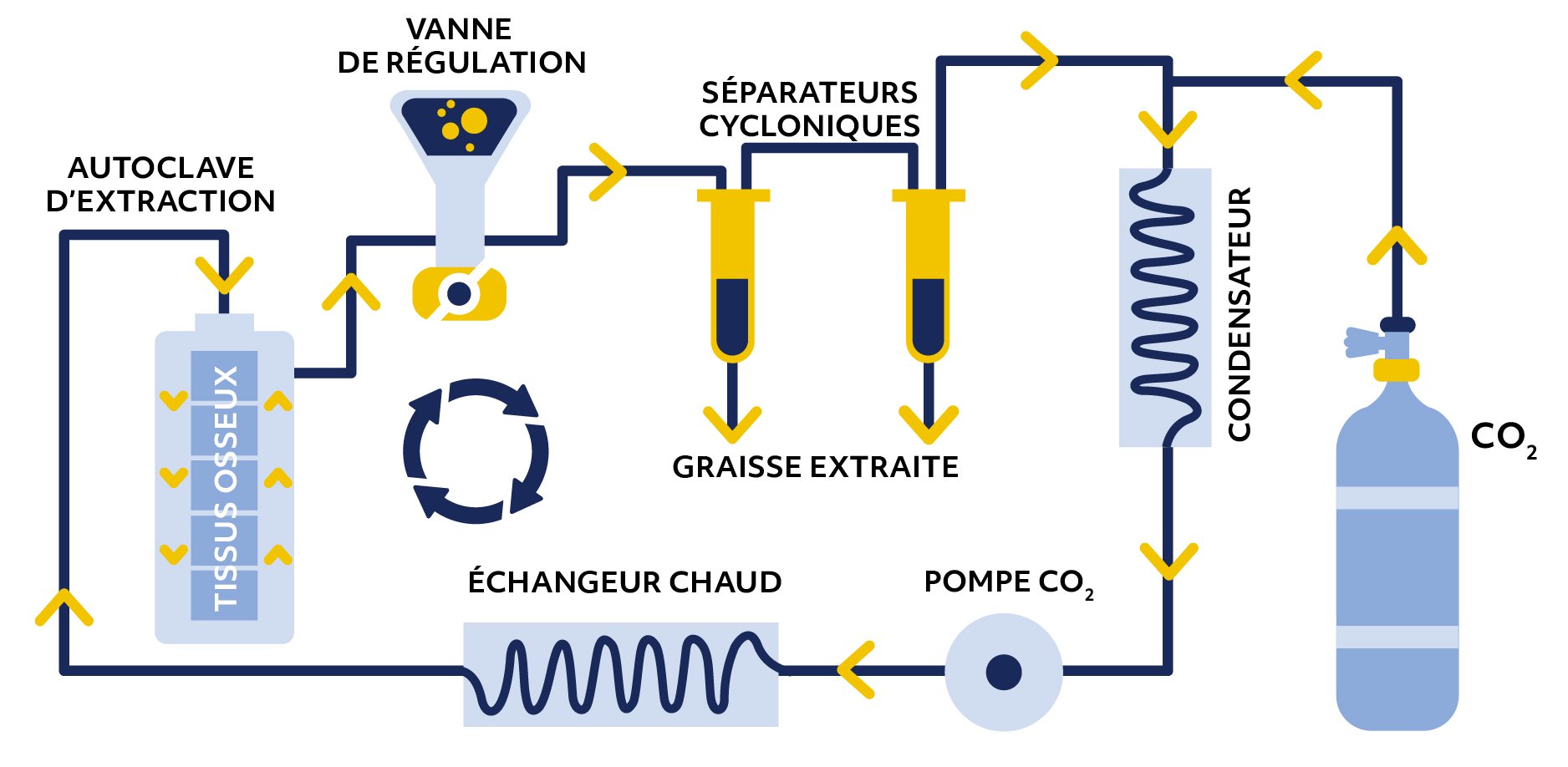
PRINCIPE ET MISE EN OEUVRE DU PROCéDé SUPERCRIT®
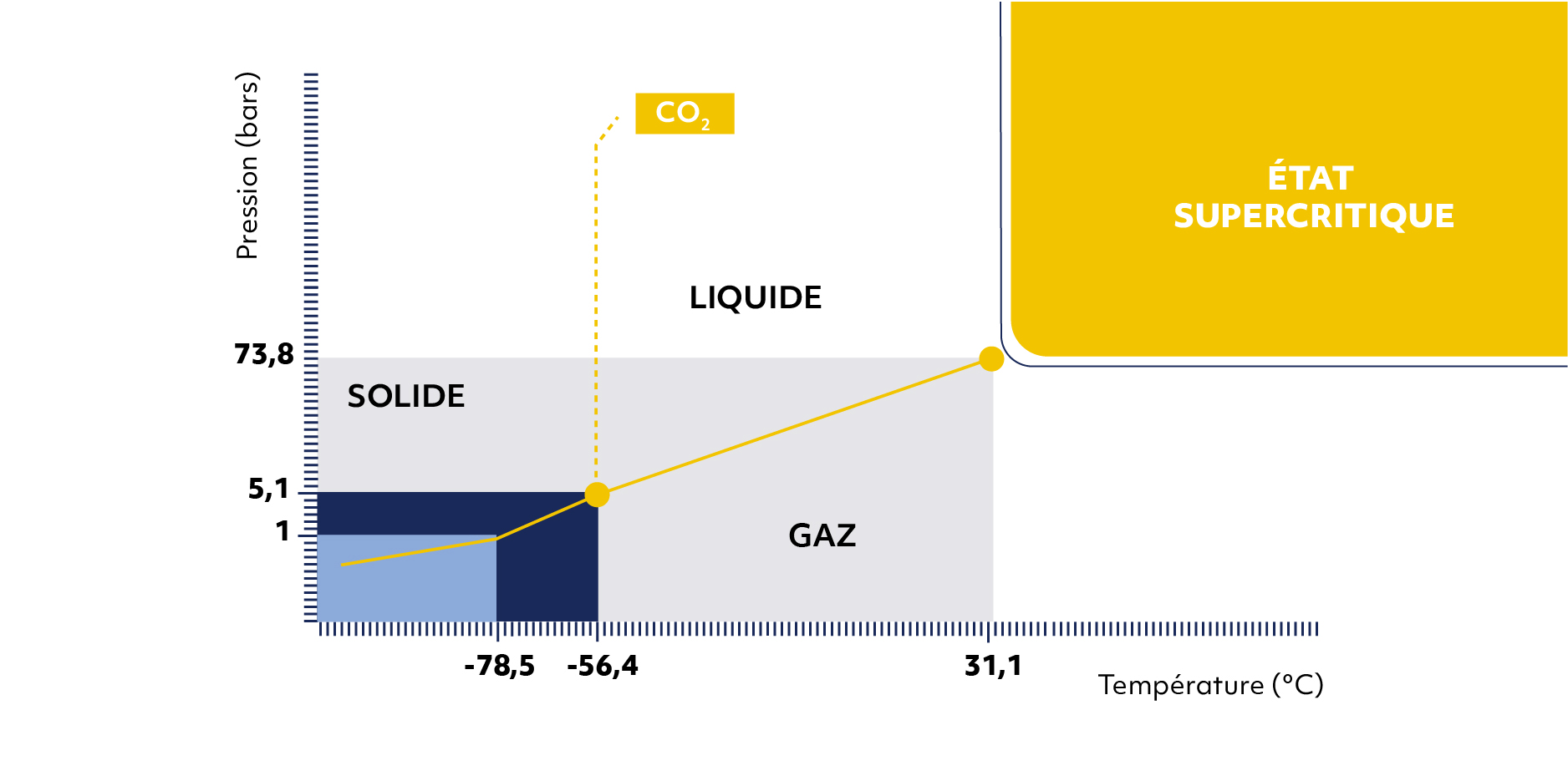
Dans son principe, au-delà d’une pression et d’une température critiques, le CO2 est dans un état intermédiaire entre gaz et liquide appelé supercritique (figure 2) qui combine un pouvoir solvant important et une très faible viscosité (8).
Ces deux propriétés rendent ce fluide très performant pour le dégraissage du tissu osseux. Le CO2 supercritique est en outre non toxique et neutre sur la trame osseuse. Il ne laisse aucun résidu en fin de traitement. Le procédé Supercrit® est la combinaison du dégraissage par le CO2 supercritique et d’une oxydation chimique en solutions aqueuses des protéines résiduelles.
La préparation des greffons est réalisée dans un environnement de haute technicité, alliant des équipements entièrement automatisés mis en œuvre par des techniciens hautement qualifiés. La supervision continue de l’environnement, les contrôles qualité à chaque étape et le suivi microbiologique des opérations garantissent la maîtrise du procédé Supercrit®.
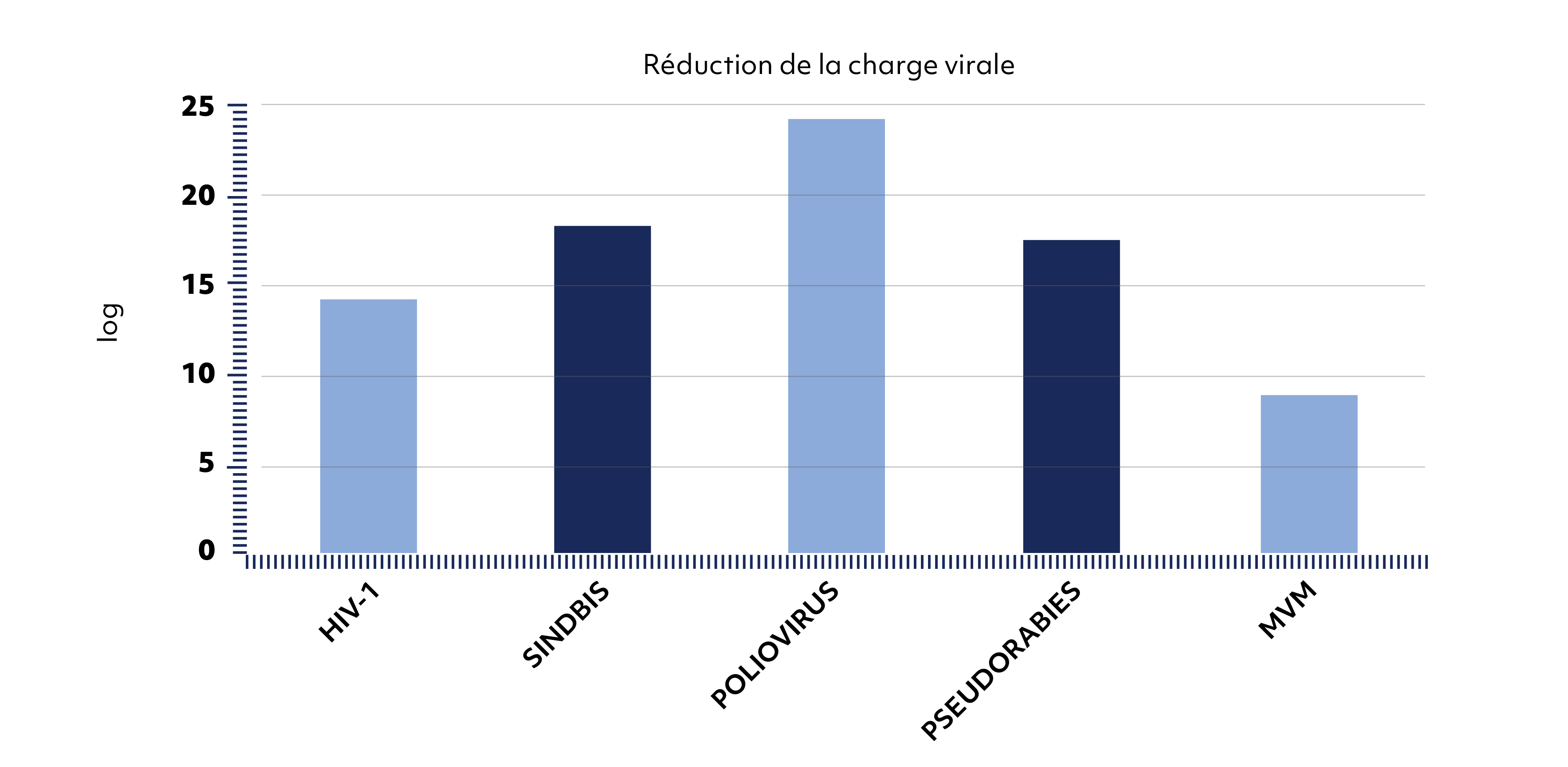
Les performances du procédé Supercrit® en termes de réduction d’une charge virale préalablement inoculée ont été validées (4, 5). Le pouvoir viro-inactivant du procédé dépasse largement le seuil d’assurance qualité (SAL) de 10-6 exigé pour assurer la stérilité des greffons osseux (Figure 3). L’évaluation de l’élasticité et de la résistance à la compression des greffons osseux BIOBank, réalisée selon plusieurs protocoles, a démontré la spécificité du procédé Supercrit® à préserver mieux que d’autres l’architecture et la densité du tissu osseux (1, 2, 3).
PROCÉDÉ SUPERCRIT® & viro-inactivation
La sécurité virale des produits thérapeutiques d’origine humaine est un enjeu fondamental pour les chirurgiens greffeurs comme pour leurs patients. Dans le but de démontrer la sécurité virale de ses greffons osseux, BIOBank a conduit plusieurs études d’évaluation de l’efficacité viro-inactivante de son procédé Supercrit® appliqué au tissu osseux.
Les premières études ont été réalisées en 1995 et 2003 par l’Institut Pasteur, référence mondiale en virologie. Elles ont démontré un pouvoir viro-inactivant très supérieur aux exigences des normes en vigueur sur toutes les catégories de virus, dans des modèles différents et complémentaires.
La dernière étude a été réalisée en 2022 par le laboratoire Texcell, spin-off de l’Institut Pasteur et laboratoire Français de renommée scientifique internationale dans le domaine.
BIOBank est à ce jour la première banque de tissus à avoir pris en considération les risques liés au virus de la Covid-19 par cette étude scientifique spécifique. Celle-ci est venue compléter les études précédentes pour constituer un ensemble de preuves scientifiques sans équivalent :
• Étude n° 1 : Évaluation du procédé Supercrit® sur l’inactivation des virus modèles classiques comme le HIV, le virus de l’Hépatite C, le virus de l’Hépatite B, le virus de l’Hépatite A et les Herpes virus (1)
• Étude n° 2 : Évaluation de l’inactivation du Parvovirus, virus modèle le plus résistant, au cœur de la tête fémorale complète, montrant la capacité du procédé Supercrit® à agir en profondeur dans le réseau trabéculaire osseux (2).
• Étude n° 3 : Évaluation de l’inactivation du Sars-CoV-2 et du West Nile Virus, permettant de démontrer l’efficacité de la viro-inactivation sur les nouveaux virus émergents responsables des pandémies et épidémies actuelles : Covid-19, Chikungunya, West-Nile, Dengue, Fièvre Jaune, Zika (3, 4).
(1) Viral inactivation of human bone tissue using supercritical fluid extraction., Fages J., Frayssinet P., Poirier B., Barbier Y., Joffret ML, Larzul D., Majewski W., Bonel G., ASAIO Journal 1998, 44 : 289-293.
(2) Evaluation of the viral safety level during manufacturing process of human bone grafts. Evaluation carried out by using the whole femoral head, Pasteur-Texcell, Report number : 250/01/5375/01, 2003.
(3) Viral clearance evaluation associated to hydrogen peroxide (H2O2) and washing treatments of the purification process of bone grafts from human origin. Evaluation carried out by using the whole femoral head, Texcell, Report number : 250/02/5870- A1/01, 2022
(4) Viral clearance evaluation associated to sodium hydroxide (NaOH) and washing treatments of the purification process of bone grafts from human origin. Evaluation carried out by using the whole femoral head, Texcell, Report number : 250/02/5871- A1/02, 2022
mode d'utilisation et mécanisme d'action des greffons osseux biobank
Le respect de l’intégrité du tissu osseux par le procédé Supercrit® a permis à BIOBank de développer une gamme complète de produits adaptés aux besoins spécifiques des chirurgiens. La conservation des greffons dans un état déshydraté requiert une phase de réhydratation préalable à la greffe pour que le tissu osseux retrouve son élasticité initiale. Celle-ci peut être réalisée avec une solution de chlorure de sodium physiologique stérile.
Comme tout biomatériau inerte, l’allogreffe osseuse BIOBank agit par ostéoconduction. La matrice osseuse greffée se laisse envahir par une vascularisation issue du lit osseux receveur qui va lui apporter les cellules osseuses nécessaires à son remaniement et à sa transformation en un tissu osseux vivant et fonctionnel (6, 7). Ce mécanisme est d’autant plus efficace que le procédé Supercrit® maintient les caractéristiques biologiques et biomécaniques du tissu osseux originel.
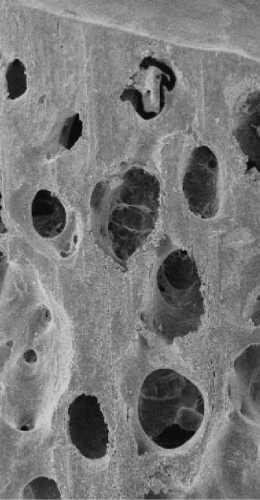
Ce qui fait la différence avec les autres matériaux ostéoconducteurs tient dans la composition intrinsèque de sa structure minéralo-collagénique, la préservation de son architecture (Figure 4) et de ses propriétés mécaniques ainsi que dans la mouillabilité particulièrement forte consécutive à l’efficacité du procédé Supercrit®.

CONCLUSION
L’ensemble des études in vitro et précliniques valident la pertinence du procédé Supercrit®. En alliant performance du nettoyage et de la viro-inactivation et respect de la trame osseuse, il constitue une approche moderne et efficace. Il convient également de retenir que la mise en œuvre d’un tel procédé se fait au sein d’une organisation complexe, nécessitant la maîtrise de nombreuses compétences et plaçant la rigueur au cœur de chacune des étapes.
Le recul clinique aujourd’hui disponible témoigne de l’intérêt des allogreffes osseuses et confirme que le tissu osseux est un biomatériau naturel exceptionnel.