Our orthopaedic and spine range
BIOBank bone grafts derive from human femoral heads which are harvested only in France and exclusively from living donors by orthopaedic surgeons during hip arthroplasties. Donor selection is based on strict compliance to health care safety rules, clinical and serological regulatory criteria. The femoral heads are then transformed into viro-inactivated, sterile bone grafts through the implementation of our exclusive Supercrit® process.

clinical evidence
The safe and effective use of supercritical CO2-processed bone allografts for cervical and lumbar interbody fusion: A retrospective study
> 16 patients underwent ACDF & 37 patients underwent lumbar ALIF
> No safety complications during surgery or post-operatively
> Radio Results: 95.3% and 90.5% of cervical and lumbar patients showed successful bone fusion, respectively.
> Patient Experience: > 80% of patients reported good to excellent outcomes according to Odom’s scores.
> Supercrit® allografts have comparable results to autografts, with added benefit of higher biological safety.
> These results convinced the authors to increase their use of allografts to 50% of procedures.


Whole femoral head
Reference
| 90001 | - Whole femoral head |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
30 minutes

Half femoral head
Reference
| 90003 | - Half femoral head |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
30 minutes

Bone wedge
References
| 90019 | - Bone wedge | 6mm | -6° |
| 900015 | - Bone wedge | 8mm | -8° |
| 900016 | - Bone wedge | 10mm | -10° |
| 900017 | - Bone wedge | 12mm | -12° |
| 900018 | - Bone wedge | 14mm | -14° |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
15 minutes

Cancellous bone block
References
| 90011 | - Cancellous bone block | 30x20x10mm |
| 90012 | - Cancellous bone block | 20x10x10mm |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
15 minutes

Cancellous bone dowels
References
| 900909 | - Cancellous bone dowels | 28x9mm |
| 900910 | - Cancellous bone dowels | 28x10mm |
| 900911 | - Cancellous bone dowels | 28x11mm |
| 900912 | - Cancellous bone dowels | 28x12mm |
| 900913 | - Cancellous bone dowels | 28x13mm |
| 900914 | - Cancellous bone dowels | 28x14mm |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
15 minutes

Cancellous bone granules in vial
References
| 90072 | - Cancellous bone granules | 7cc |
| 90074 | - Cancellous bone granules | 18cc |
| 90075 | - Cancellous bone granules | 25cc |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
10 minutes
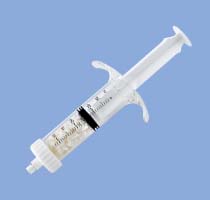
Cancellous bone granules in syringe
References
| 90082 | - Cancellous bone granules | 7cc |
| 90084 | - Cancellous bone granules | 18cc |
| 90085 | - Cancellous bone granules | 25cc |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
10 minutes

Cancellous bone powder in syringe
References
| 90035 | - Cancellous bone powder | 0.5cc |
| 90036 | - Cancellous bone powder | 1cc |
| 90037 | - Cancellous bone powder | 2cc |
Indications
The therapeutic indications for the BIOBank grafts are filling and structural grafts in :
- Segmental substance loss during arthroplasties revisions
- Curettage of benign bone tumours
- Fracture with osteosynthesis
- Osteotomy with osteosynthesis
- Spinal arthrodesis
Hydration
Few minutes



